North America, Europe Lead Lyophilization Development
North America, Europe Lead Lyophilization Development

North America and Europe are anticipated to be the most lucrative regions in the global lyophilization market owing to the presence of biotechnology and pharmaceutical companies and the increasing presence of CDMOs and CMOs in the regions. Both North America and Europe have a high adoption rate of lyophilization services and products due to the increasing number of pharmaceutical and biopharmaceutical companies in those regions.
Market Breakdown
North America
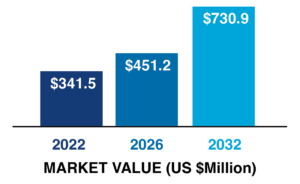
North America is the most lucrative market in the global lyophilization market with $322 million in revenue in 2021 due to high product demand in the pharmaceutical and food processing industries. Fact.MR forecasts the North American lyophilization market to grow from $341.5 million in 2022 to $730.9 million by 2032. By product type, the freeze-dryer segment will dominate the North American lyophilization market in terms of revenue in 2022, and this trend is projected to continue throughout the forecast period.
North American Lyophilization
Market Value Analysis, 2022-2032
Europe
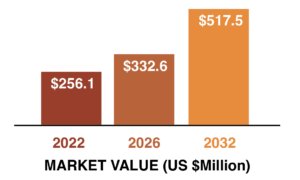
Fact.MR forecasts the Europe lyophilization market to grow from $256.1 million in 2022 to $517.5 million by 2032. The Europe lyophilization market represent absolute revenue opportunity of $15.7 million in 2023 over 2022 and incremental opportunity of $261.4 million between 2022 and 2032.
By product type, the freeze-dryer segment is estimated to hold the highest market share of 68.2% in the year 2022. Germany is estimated to hold for 23% of the total market share in 2022, and biopharmaceutical companies are expected to have the highest market share of 35.1% in 2022.
European Lyophilization Market Value
(US $ Millions) Analysis, 2022-2032
Lyo Market Growth Depends on Regulations
There are stringent GMP guidelines for the manufacture of freeze-dryers. Because lyophilization equipment stabilizes drug molecules used for improving human health or animal health, it is necessary to follow the guidelines from various regulatory bodies like the Food & Drug Administration (FDA) and European Regulatory bodies. The growth of this market is very much dependent on these regulatory guidelines.
FDA Manufacturing Guidelines
- GLP/GMP compliance means having a validated designing, manufacturing, and testing facility for pharmaceutical and biopharmaceutical products. These guidelines contain the validation of the lyophilization process and equipment. As per these guidelines, the lyophilization equipment requires Performance Qualification.
- FDA governs all inspection guidelines for the lyophilization of drug products. The Lyophilization of Parenterals (7/93) is the guide for the lyophilization of parenterals. This references material for investigators and other FDA personnel.
- The Center for Biologics Evaluation and Research (CBER) of the FDA regulates freeze-dried biological products in its section pertaining to residual moisture, as published in Title 21 of the Code of Federal Regulations for Food and Drugs.
FDA Regulatory Considerations for Pharmaceutical Lyophilization
- Hold Time and Equipment Compatibility
- Fill Volume/Fill Volume Tolerances
- Product-specific Characterization/Development Studies
- Establishing the Critical Process Parameters
- Establishing the End Points for Primary and Secondary Drying
- Sampling Plan
- Critical Quality Attributes
- Scale-up
- Post-Approval Lifecycle Perspective
European Union Regulations
- The European Medicines Agency (EMA) coordinates inspections to verify compliance with these standards and plays a key role in harmonizing GMP activities at the European Union (EU) level.
- Any manufacturer of medicines intended for the EU market, no matter where in the world it is located, must comply with GMP.
- Annex 17: Real Time Release Testing and Parametric Release provides guidance for the interpretation of the principles and guidelines of good manufacturing practice (GMP) for medicinal products as laid down in Directive 2003/94/EC for medicinal products for human use and Directive 91/412/EEC for veterinary use.
- Process Analytical Technology (PAT)/RTRT (Real Time Release Testing, Annex 17 of the EU GMP Guide) systems for in-line process monitoring are used to control and determine critical processing parameters. PAT plays an important role in continuous lyophilization processes.
- In February 2020, the Directorate for Health and Food Safety of the European Commission published a second draft for the revision of Annex 1 of the EU GMP Guide. Chapter 8, “Production and Specific Technologies,” discusses approaches to the sterilization of products, equipment, and packaging components. The chapter now also discusses different technologies, such as lyophilization, form-fill-seal, and single-use systems (SUS), where specific requirements apply.
Partnering with a CDMO that understands the various lyophilization regulations will be essential in accelerating the development of complex drug candidates.
References
Talk to a Pii Scientist Today About Your Stand-Alone Contract Analytical Needs
ABOUT Pii
Pharmaceutics International, Inc. (Pii) is a US-based contract development and manufacturing organization (CDMO) located in Hunt Valley, Maryland. The experienced scientists, engineers, and staff at Pii pride themselves on adroitly employing a phase appropriate method of drug development for the prudent use of their customer’s resources as they solve challenging problems. In addition to offering end-to-end development services, Pii manufactures a variety of dosage forms to include complex parenteral drugs and has a wealth of analytical testing capabilities. Its Hunt Valley campus has four aseptic suites with lyophilization capabilities. Our talented professionals stand ready to help!






