Contact Us
- Solutions
- Resources
- About
- Contact Us
close
Optional callout banner for highlighted news or events
Learn More

Custom lyophilization services and formulation development.
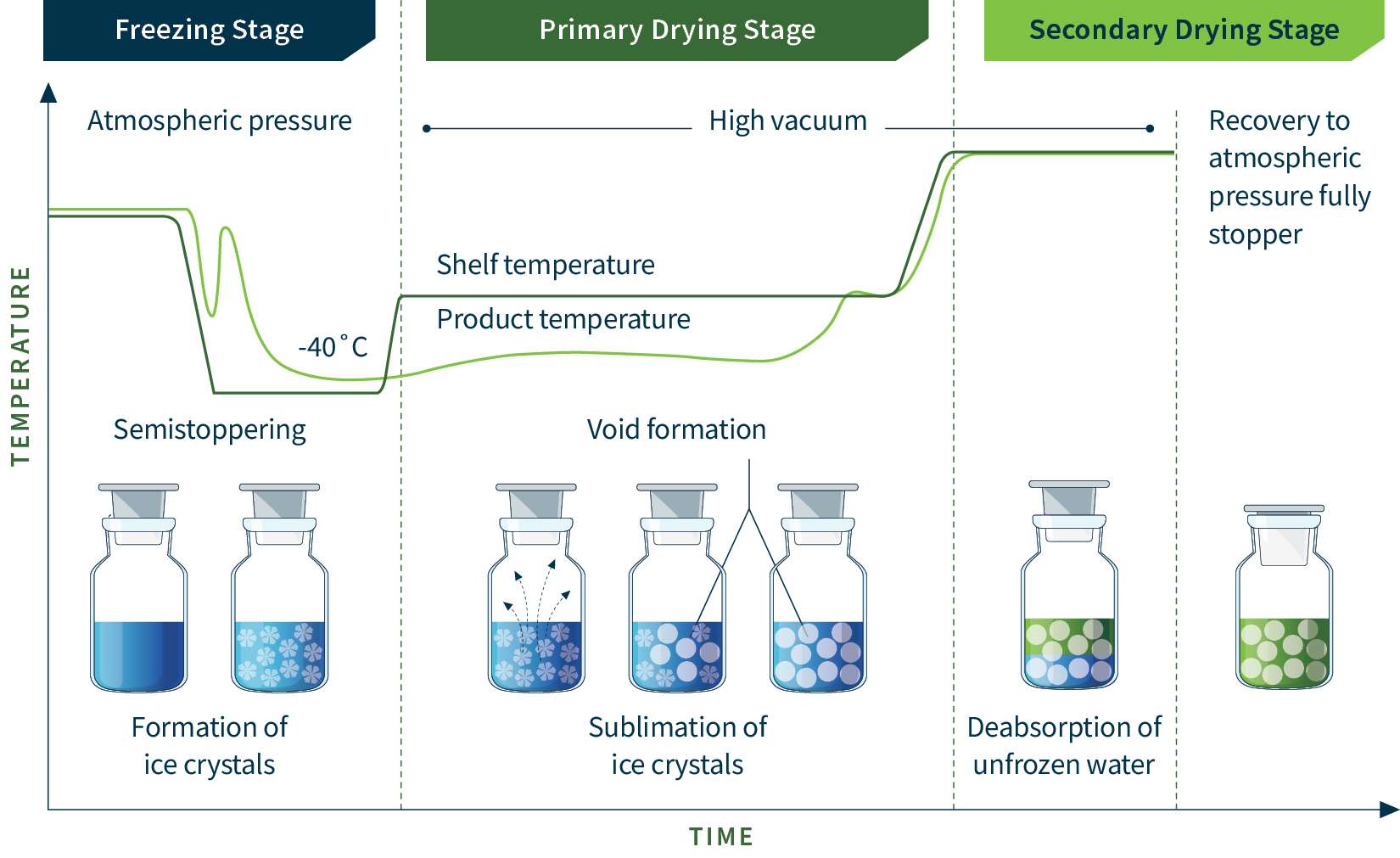
Expert-led lyophilization cycle development for success.
Clinical Trial Supply without the waste, providing 3mL to 50mL vials.
Commercial batch sizes up to 20k units.