Contact Us
- Solutions
- Resources
- About
- Contact Us
close
Optional callout banner for highlighted news or events
Learn More
Last year marked the golden anniversary of the National Cancer Act, landmark legislation committed to fighting cancer. Unfortunately, the demand for oncology drugs is expected to rise due to an increasing prevalence of cancer cases and a surge in the geriatric population globally. The business is expected to see more growth in the coming years due to the growing R&D initiatives of the players engaged in the industry.
Oral administration is an appealing route of delivering cancer treatments. However, the gastrointestinal tract is characterized by specific and efficient physical, chemical, and biological barriers that decrease the bioavailability of medications, including chemotherapeutics. Compared with pills and tablets, a more efficient way of getting drug into the blood is to inject it directly into a vein. This way, all the drug gets circulated throughout the body and avoids degradation in the stomach.1
Parenteral drug delivery allows direct administration of drug substances and ensures bioavailability. Parenterals are sterile preparations containing one or more active ingredient for administration by injection or infusion. Of the $531.8 billion global parenteral drug sector, oncology represents almost 13% of that market.2
Since President Nixon signed the National Cancer Act, there have been incredible advancements in how cancer is detected, treated, and cured. One of the most promising is targeted therapy, which is the foundation of precision medicine. This treatment targets proteins that control how cancer cells grow, divide, and spread. Targeted treatments can control drug dosing.
The global parenteral drug market is dominated by large molecules. These biological injectable preparations for treating cancer include:

Targeted drug delivery can be used to increase the therapeutic index by protecting normal cells from damage and preventing drug resistance. For example, compared with conventional antitumor drugs, drug delivery systems such as drug nanoparticles (NPs) are expected to have more advantages in antineoplastic effects, including easy preparation, high efficiency, low toxicity, especially active tumor-targeting ability.5 The increased popularity of targeted oncology drugs with the ability to treat multiple cancer types is projected to fuel demand growth.
Targeted nanoscale drug delivery technologies are particularly suited for hard-to-treat cancers – mesothelioma, pancreatic cancer, and glioblastoma (a fast-growing and aggressive brain tumor) – as the drugs can be delivered exactly where they are needed and nowhere else. Nanogels injected onto the cavity surface of the brain after a tumor has been removed, gradually release anti-cancer drugs to eliminate any residual tumor cells. Combining gels and molecular vehicles enables opportunities to enhance therapies and control delivery timescales.6
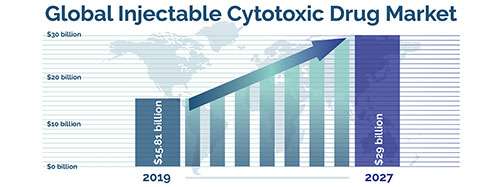
Injectable Cytotoxic Drugs Market Size, Share & COVID-19 Impact Analysis, Fortune Business Insights, Jan. 2021.
Researchers continue to explore how to control delivery and release cytotoxic payloads at the site of disease, sparing normal organs. The global injectable cytotoxic drugs market was $15.81 billion in 2019 and could reach $29 billion by 2027.7 The crucial role played by parental administration of cytotoxic drugs in countering the cancer cells has helped in the growth of this segment.
Industry is also exploring the benefit of long-acting injectables (LAIs). Such formulations release a compound over several weeks to provide a more effective treatment for chronic conditions, reducing dosing frequency and improving patient compliance. These tend to be intramuscular injections.

According to Fact.MR, outsourcing is expected to be more prominent in oncology. This is primarily driven to an increase in number of large molecule drugs that need to be manufactured in injectable format.
“Today’s global pipeline is dominated by complex oncology products, while sales of rare disease treatments are predicted to double by 2026 – to up to $268 billion. Often based on highly sensitive molecules and delivered parenterally, these new products have fueled surging demand for technically sophisticated manufacturing support.” 8
This CDMO support begins with early clinical trials of precise and targeted drugs – priority research for the National Cancer Institute as it looks to future treatments 50 years after the signing of the National Cancer Act.
Like what you read? Share with your network: